Last updated January 24, 2018 at 4:56 pm
It’s one of the great mysteries – where did the organic molecules that would be the ingredients for life on our young Earth come from? One possible source is becoming increasingly plausible thanks to new research released this week, that those basic organic molecules formed in space itself.

Asteroids and comets like 67P/Churyumov–Gerasimenko could have been the source of organic molecules leading to life on Earth.
Credit ESA/Rosetta/NAVCAM
Throughout space there are lumps of ice packed with chemical molecules, called molecular ices. Formed due to the low temperature of space, gases such as methane and oxygen condense onto surfaces and become trapped as solid ice around dust grains, comets and asteroids.
Around 4 billion years ago, some of these frozen molecular ices are thought to have crashed into a young Earth during a period of heavy bombardment by asteroids, potentially delivering organic molecules in the ice and sparking off a complex process that led to the development of life.
Organic molecule factories
What led to these frozen gases reacting and forming the more complex organic molecules within the molecular ices?
According to Canadian researchers, radiation can make these ices into organic molecule factories, acting as the catalyst for chemical reactions between the methane and water.
If this same mechanism also occured in the vacuum of deep space, it could well have resulted in the formation of the first organic molecules that eventually crashed to Earth.
To confirm this idea, the researchers created an artificial space environment on Earth. Inside a vacuum chamber, frozen ice containing methane and oxygen was bombarded with beams of electrons.
This mimicked the conditions in space, where molecular ices are subjected to multiple forms of radiation, including X-rays. As this radiation hits matter, it knocks free secondary electrons called low energy electrons.
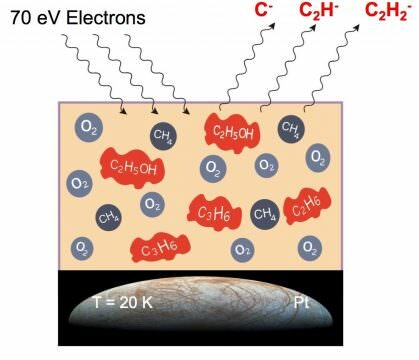 Using beams of these low energy electrons, the researchers found that reactions within the frozen methane produce a variety of small organic molecules including propylene, ethane and acetylene.
Using beams of these low energy electrons, the researchers found that reactions within the frozen methane produce a variety of small organic molecules including propylene, ethane and acetylene.
When methane was mixed with oxygen in the frozen ice, ethanol was formed. In addition, there were traces which would suggest methanol, acetic acid and formaldehyde were also being produced.
“Low temperatures that are just a few tens of degrees above absolute zero should slow chemical reactions down to near-stillness,” said Swinburne University of Technology astronomer Professor Alan Duffy, who wasn’t involved in the research.
“The emptiness of space means random chemicals should rarely if ever meet. The electrons act to catalyse, or speed up, these reactions while the ices act as a way to bring the chemicals close enough to one another for the reactions to occur”
Similar results were also found when X-rays were used directly on the ice, and other studies have shown a similar effect using ultraviolet or other types of radiation.
The results suggest that it is not only the influence of the radiation itself, but the secondary electrons caused by the radiation hitting the ice that results in the chemical reactions occurring.
 If correct, this research helps clarify a key part of the development of life on Earth. In an early universe, these molecular ices were bombarded by radiation which freed electrons to react oxygen and methane into more complex organic molecules.
If correct, this research helps clarify a key part of the development of life on Earth. In an early universe, these molecular ices were bombarded by radiation which freed electrons to react oxygen and methane into more complex organic molecules.
These molecular ices then crashed to Earth, some of which fell into pools of water, which provided the ideal conditions of drying and wetting to cause further reactions and build larger and more complex molecules.
Eventually these molecules called nucleotides combined into long chains, which along with phosphorylating agents combined to form RNA, and eventually, basic life that would one day evolve into you.
“The origin of organic chemicals, the ingredients of life, is one of the most important questions in our own origin story,” according to Duffy. “It also can tell us how common these ingredients around other young alien worlds and perhaps help us begin to answer that other great question – are we alone?”
The research has been published in Journal of Chemical Physics
Follow us on Facebook, Twitter and Instagram to get all the latest science.