Last updated January 19, 2018 at 1:32 pm
Researchers have developed the first successful method for growing functional human muscles from stem cells, opening up a world of possibilities for the research and treatment of rare muscular diseases.

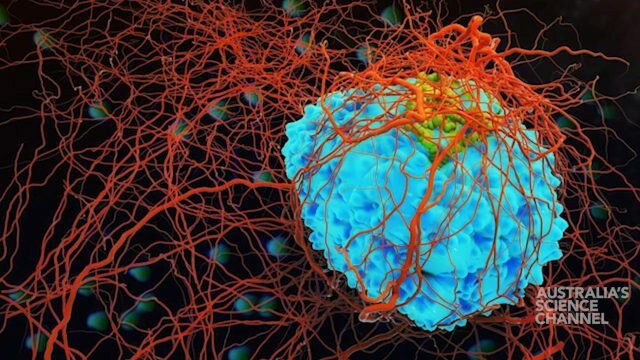
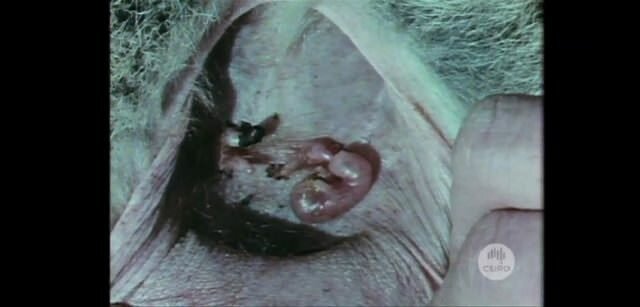
Lab-grown human muscle fibres
Move aside Dwayne Johnson, there is a new muscle-man in town. His name is Nenad Borsac, and together with a team of researchers, he has developed a way of growing far more muscle than The Rock could ever hope for.
They began with human skin cells, which they then converted into pluripotent stem cells – cells which can be induced to form nearly any tissue type in the body. The induced pluripotent stem cells were then grown while being flooded with a molecule called Pax7 – which signals the cells to start becoming muscle.
As the cells grew and divided, they became very similar to adult muscle stem cells. Previous researchers had reached this point, but had never been able to progress to fully-formed muscle cells.
Bursac and his team succeeded in that final step by growing the cells on a 3 dimensional scaffold which allowed them to form aligned and functioning human muscle fibres. Additionally, when the cells began forming muscle fibres on the scaffold, the researchers stopped providing Pax7 and started giving the cells a different growth media, which allowed them to fully mature into muscle cells.
The result was a fully functioning, lab grown human muscle.
“It’s taken years of trial and error, making educated guesses and taking baby steps to finally produce functioning human muscle from pluripotent stem cells,” said Lingjun Rao, a postdoctoral researcher in Bursac’s laboratory. “What made the difference are our unique cell culture conditions and 3-D matrix, which allowed cells to grow and develop much faster and longer than the 2-D culture approaches that are more typically used.”
The new muscles, however, are not as strong as native muscle tissue.
Despite this limitation, being able to start from cellular scratch using non-muscle tissue will allow scientists to grow far more muscle cells, which they hope will assist research into genome editing and cellular therapies. It is also hoped it will allow the development of individually tailored models of rare muscle diseases for use in drug discovery and basic biology studies.
This is not the first time the group has grown muscles from cells. Previously, Bursac and his team has started with samples of cells taken from muscles via biopsy. These cells, called myoblasts, had progressed beyond a stem cell state but hadn’t fully become muscle cells, and using a similar scaffold method had been grown into functioning muscles.
However, the new study growing the cells from pluripotent stem cells is several steps more advanced. Not only did they start with the non-muscle cells, but the muscles developed from the stem cells also included reservoirs of “satellite-like cells” that are necessary for normal adult muscles to repair damage. The muscles developed from biopsied myoblasts had much fewer of these cells.
The stem cell method is also capable of growing many more cells from a smaller starting batch than the biopsy method.
“The prospect of studying rare diseases is especially exciting for us,” said Bursac. “When a child’s muscles are already withering away from something like Duchenne muscular dystrophy, it would not be ethical to take muscle samples from them and do further damage. But with this technique, we can just take a small sample of non-muscle tissue, like skin or blood, revert the obtained cells to a pluripotent state, and eventually grow an endless amount of functioning muscle fibers to test.”
The research was published in Nature Communications
Video and image courtesy of Nenad Bursac and Duke University.